by David Gardner, Chemical and Biomolecular Engineering
Teaching Effectiveness Award Essay, 2017
Challenge: Thermodynamics is a notoriously difficult subject. It’s no surprise that the subject causes anxiety and animosity – even physicist Arnold Sommerfeld was quoted as saying that even by the third time you learn thermodynamics, “you know you don’t understand it, but you’re so used to it, it doesn’t bother you.” Despite its reputation, however, learning the subject is essential to succeeding in upper-level engineering classes and beyond. Thermodynamics is the rule book that decides which chemical processes are feasible and which are a waste of resources. In week 6 of Intro to Chemical Engineering, we introduce (mostly apprehensive) freshmen to the basics of thermodynamics. From a previous survey of my discussion section, I knew two things: the students were dreading thermodynamics, and that they were interested in green technologies.
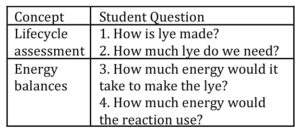
Solution: I brainstormed lesson plans to make the material have a personal connection, and recalled a seminar where former Dow CTO and current UW Madison professor Dr. Bill Banholzer used engineering principles to evaluate real proposals that had come across his desk for reducing Dow’s greenhouse gas emissions [1]. I decided to adapt his seminar to show how useful thermodynamics was to engineers in the real world. I spent the first five minutes of my discussion section introducing one of the proposals. The company, Skyonic, proposed to used lye (NaOH) to convert CO2 from a Dow power plant to baking soda. Then I broke the students into groups to pretend they were the CTO and had to evaluate this proposal: what questions would they ask? I walked around to each group, and after ten minutes, I brought the class together to talk about the process from a thermodynamic point of view. Below is a sample of questions the students came up with, sorted by concept, and the calculation that I showed the students how to work through on the board.
Tethering the abstract laws of thermodynamics to a real-world example helped students “connect the dots” and got the students engaged with the material; using the rule-book of engineering saved this company from a disastrous project that would have failed its environmental goal. The activity generated a discussion about alternative energy and carbon storage, with some students emailing me after class to learn more.
Assessment: I evaluated the activity by (1) student engagement during the activity, (2) changes in student attitudes towards thermodynamics before and after the activity, and (3) ability to solve energy balance problems. The activity succeeded by the first standard. The second standard was measured by a weekly survey I send out to gauge students’ feelings about the material, and the fraction of students who rated energy balances as confusing dropped from 13/25 to 4/25. The final standard was evaluated by small-group work on an energy balance problem in-class, which six of the eight groups could do. This real-world example of applying thermodynamics to critically evaluate projects improved student engagement, which lead to improved levels of competency solving energy balance problems.
References:
[1] http://banholzer.che.wisc.edu/presentations/UW-CBE-Seminar.pdf